Overview -
ECMO made affordable
About ECMO
ECMO or Extracorporeal Membrane Oxygenation is a type of Mechanical Circulatory Support device that performs the role of the heart and lungs externally. Blood is taken out of the body, it goes through the ECMO machine that oxygenates the blood with the help of an oxygenator and it is then sent back into the body and pumped to the different organs. A person is put on ECMO for one of two reasons — when there is a heart failure/pump failure or when there is a lung failure. The ECMO machine is controlled by a perfusionist or a nurse with advanced training in ECMO.What are the uses of ECMO?
ECMO can be used as a bridge to transplant. This is used for patients with heart failure who are awaiting a heart transplant. Once the transplant is performed, the patient is taken off ECMO. It can also be used as a bridge to definitive therapy. Patients with bad lungs following a severe infection are put on ECMO to give the lungs a rest till it can recover. Once the lungs recover, they are taken off ECMO.How does an ECMO machine work?
The ECMO machine is connected to a patient through cannulae (plastic tubes). The tubes are placed in the large veins and arteries in either the leg, neck or chest. The ECMO machine now pumps the oxygen-low blood from the patient’s body to an artificial lung (oxygenator). This oxygenates the blood and removes the carbon dioxide, just like our lungs. Once done, the ECMO machine sends the blood back to the patient via a pump with the same force that the heart would normally exhibit. Based on the condition of the patient, the settings on the machine can be adjusted to give them the required heart and lung support.How is the cannulation done?
Previously, surgeons used to open the chest (sternotomy) and put one cannula into the venous system or the right atrium from where the blood is taken out of the body and another cannula into the aorta where the oxygenated blood is returned to the body. But nowadays, with modern technology, we do what is called percutaneous peripheral ECMO. In percutaneous peripheral ECMO, the cannula is put into the femoral vein. This drains the blood, it gets oxygenated and then it is given back to the femoral artery in the groin (VA-ECMO) or jugular vein (VV-ECMO).What are the types of ECMO?
There are two types: 1. Venoarterial (VA) ECMO and 2. Venovenous (VV) ECMO VA-ECMO can be used for heart and lung support, while VV-ECMO is used for lung support only. In VA-ECMO, venous blood from the inferior vena cava (a large vein that carries deoxygenated blood from the lower body into the right atrium of the heart) is drained through a cannula often placed in the femoral vein. The blood passes through the oxygenator where it is oxygenated before being sent back to the arterial system through the femoral artery. In VV-ECMO, blood from the inferior vena cava is drained through a cannula often placed in the femoral vein. The blood passes through the oxygenator and it is then returned to the venous system through the internal jugular vein.Introduction to VA-ECMO
VA-ECMO is a temporary mechanical circulatory support system that enables complete and immediate cardiopulmonary support in the setting of cardiogenic shock and cardiac arrest (53). It consists of a centrifugal pump capable of propelling up to 8 L/min of blood and venous drainage and arterial return cannulas. A hollow fiber membrane oxygenator is spliced into the circuit that not only provides blood oxygenation but also carbon dioxide (CO2) clearance via sweep gas flow. This latter function is a critical distinguishing feature from other MCS strategies, such as IABP and pVADs. VA-ECMO may also be placed surgically, especially in the post-cardiotomy setting, when oxygenated blood is returned directly into the ascending aorta (central cannulation technique). However, this review focuses primarily on the use of peripherally placed VA-ECMO as this is the most common type of support instituted by cardiologists in the setting of cardiac arrest or refractory CS. The preferred approach for percutaneous VA-ECMO is femoral artery and vein cannulation. In an adult, the tip of an 18–28 Fr cannula draining deoxygenated venous blood is positioned in the mid right atrium (RA) or the superior vena cava-RA junction. After passing through the “membrane lung,” oxygenated blood is returned to the systemic circulation via a 15–19 Fr arterial cannula with its tip typically positioned in the iliac artery. Selecting cannulas with appropriate diameters is critical not only to reduce the risk of vascular injury but also to avoid significant negative inflow (preferably <50 mmHg) and high outflow pressure (<300 mmHg). To mitigate the risk of distal limb ischemia, an 8 Fr distal reperfusion cannula is routinely inserted into the superficial femoral artery in our center and is spliced into the arterial limb of the circuit (2, 54). Peripheral VA-ECMO is increasingly utilized as a short-term support strategy to manage patients presenting with cardiac arrest, severe biventricular HF and CS stages C-E, independent of etiology (48). It can be initiated safely in the cardiac catheterization laboratory by experienced interventional cardiologists with very short door to support time, even during ongoing cardiopulmonary resuscitation (CPR) (55, 56). Depending on local institutional policies and the specific clinical scenario, it may also be instituted in the field (mobile ECMO programs), at bedside in the ICU, or in the operating room (57). Full VA-ECMO support not only allows time to perform diagnostic and therapeutic interventions while maintaining appropriate hemodynamics and gas exchange, but also provides time for potential organ recovery. Multiple clinical trials are currently ongoing with the aim to address the potential clinical benefits of early VA-ECMO initiation in various patient populations (4, 25).Hemodynamic Aspects of VA-ECMO Support
VA-ECMO is used in the management of CS due to its capability to reduce myocardial work (pressure-volume area) while providing complete hemodynamic and respiratory support. Myocardial pressure-volume area can be thought of as the sum of myocardial potential energy and myocardial stroke work (58, 59). Both are thought to be increased profoundly in CS due to a vicious cycle of maladaptive neurohormonal and vascular mechanisms (8, 60). In the typical VA-ECMO setup in CS, the venous inflow cannula drains blood directly from the vena cavae or the RA. This significantly decreases right ventricular (RV) preload, trans-pulmonary blood flow and, therefore, left ventricular end-diastolic volume (LVEDV) and pressure (LVEDP) (61–63). Thus, VA-ECMO likely promotes hemodynamic stabilization in the setting of CS and cardiac arrest via reduced LVEDV and LVEDP. It follows, then, that VA-ECMO has been shown to reduce stroke work in pre-clinical models of CS caused by acute myocardial infarction (64). The myocardial pressure-volume area and myocardial potential energy may be further reduced by the weaning of inotropic and vasopressor drugs once VA-ECMO support is instituted. These pharmacologic agents are known to increase myocardial oxygen consumption and left ventricular (LV) stroke work dramatically (59, 65). The use of VA-ECMO also improves systemic perfusion. Typically, mean arterial blood pressure rises after VA-ECMO initiation while the high-volume venous displacement from the RA reduces central venous pressure. The systemic arterio-venous pressure gradient increases as a result, thereby enhancing systemic circulation. This may be particularly relevant to improving blood flow in organs with portal circulation, such as the liver and kidney (63). Fluid removal and relief of venous congestion can be further enhanced by splicing a continuous veno-venous hemodialysis machine (continuous renal replacement therapy; CVVHD) into the VA-ECMO circuit. By providing large volume oxygenated blood flow, organ perfusion can be supported irrespective of the intrinsic cardiac function. Importantly, the native right ventricular function is not as critical to the provision of systemic perfusion (as is the case with IABP and some pVADs) due to the lessened reliance on transpulmonary flow with VA-ECMO. Despite the acknowledged benefits of VA-ECMO, there are still several critical gaps in the literature regarding the hemodynamic implications of prolonged VA-ECMO usage. Most notably, there is an absence of data using invasive ventricular catheterization to define how myocardial work and overall pressure-volume area is affected in the clinical (human) setting. Currently, most published pressure-volume loop data demonstrating the effects of varying levels of VA-ECMO support are based on computer simulations or animal experiments, rather than actual patient data (59, 66–68). Many of these studies used at least one fixed parameter (e.g., LV contractile strength) when performing their analysis. Yet, in real-life, these variables are interdependent and contractile strength will vary based on the Frank-Starling equation. Moreover, it is unclear how the hemodynamic responses on VA-ECMO support differ between patients with normal and depressed baseline LV ejection fraction, normal and dilated LV cavity and/or right ventricular dysfunction. Presumably, there is a diverse array of hemodynamic mechanisms in these HF sub-types, all of which remain largely uncharacterized in vivo. The effect of retrograde arterial flow on LVEDP/LVEDP and LV unloading remains controversial and deserves special mention. Some commentators argue that the retrograde blood flow increases LV afterload by increasing mean arterial BP. This is thought to raise LVEDP, decrease stroke volume, reduce native cardiac output, and render a deleterious effect on LV performance (66, 68, 69). It is likely that this phenomenon is more pertinent to patients with the complete lack of or minimal cardiac contractility, as opposed to patients that have preservation of LV function (63). Nevertheless, it is increasingly common to utilize one of the “LV venting” strategies, such as an IABP or Impella, despite unclear universal benefit (70). The device choice is often dependent on the center’s experience and the benefit of upgrading from one strategy to another remains unexplored. The populations in which venting devices offer a clear benefit remain largely uncharacterized. The hemodynamics of patients with different HF phenotypes are likely to respond differently to VA-ECMO support, thus creating a differential risk-benefit ratio for the addition of an unloading strategy. Patients with acute CS in the setting of severe, pre-existing HF and elevated left atrial pressure may be best suited for unloading. Moreover, patients with biventricular shock in whom the RV recovers before the LV, may also benefit from unloading. Under these circumstances, the RV may provide increased trans-pulmonary flow prompting a rise in LV preload, despite ongoing VA-ECMO support. The combination of increased preload and afterload may lead to an increase in the LV’s myocardial oxygen consumption, thereby supporting the need for an unloading strategy.Common Indications for VA-ECMO Support
Cardiogenic Shock Complicating Acute Myocardial Infarction Despite the widespread use of early revascularization strategies, 6–10% of patients with acute coronary syndrome will progress to develop CS, representing 60–80% of all CS cases (12, 14, 15, 71). Myocardial ischemia and necrosis may continue following the index injury as the infarct extends circumferentially and toward the subepicardial regions. This prompts a further decline in cardiac function, increase in filling pressures, and excess oxygen consumption of the healthy residual myocardium. These, combined with reduced coronary perfusion pressure, initiate a vicious cycle until ~50% of the functional LV mass is lost and CS ensues. Initiating VA-ECMO early in this setting reduces cardiac work, myocardial oxygen consumption and improves coronary blood flow. Therefore, VA-ECMO may limit infarct extension and allow time for the hibernating myocardium to recover (72). The in-hospital mortality of post-MI patients with CS approaches 70–80% with traditional management, including vasoactive agents and IABP (12, 16, 17). Several non-randomized trials have demonstrated a clear benefit of VA-ECMO support in this population. As a result, its use has increased over 5-fold between 2000 and 2010 in one report (73). In a single-center retrospective study of 98 patients with MI, early VA-ECMO cannulation was associated with an all-cause in-hospital mortality of 67.3%. Patients presenting with CS as well as cardiac arrest were included (74). In a single center, retrospective observational study, Pozzi et al. identified 56 post-MI patients who presented with evidence of CS and were supported with VA-ECMO for a mean of 8.7 days. Survival to hospital discharge reached 41.1 and 32.1% were alive after a mean follow-up of 38.0 ± 29.9 months (27). In another single center study from Korea, 20 patients post-MICS were initiated on VA-ECMO before proceeding with coronary revascularization. Although CPR was performed in 70% of the cohort before cannulation, the in-hospital survival rate reached 50% (75). Multiple other, relatively small studies from around the world have reported similar rates of successful VA-ECMO decannulation and hospital discharge in the setting of post-MI CS (10, 23, 24, 30, 33, 34, 76–78) (Table 2). Table 2 Outcomes of VA-ECMO support stratified by the initial cause of cardiogenic shock.|
Indication for VA-ECMO support |
Reported survival (%) |
|
Acute myocardial infarction |
33.8–66.7 |
|
Cardiomyopathy |
35.7–57.0 |
|
COVID-19 infection |
0–36.6 |
|
eCPR |
8.8–54.0 |
|
Fulminant myocarditis |
60.0–74.0 |
|
Primary graft failure post heart transplantation |
50.0–84.2 |
|
Massive pulmonary embolism |
38.5–53.1 |
|
Cardiomyopathy in the setting of sepsis |
59.8–75.0 |
Patient assessment prior to cannulation
Candidates for VV-ECMO are typically severely hypoxemic and/or hypercapnic and unresponsive to optimal medical management, including protective ventilation with low-tidal volumes (11) and plateau pressure less than 28–30 cmH2O (12), high levels of PEEP (13), prone positioning (14), neuromuscular blockers (15) and/or other adjunctive therapies, including nitrous oxide (16) or almitrine (17). The recent literature suggests that a PaO2/FIO2 ratio of 70–80 mmHg, Murray score >3, and pH <7.2 provide a reasonable threshold for considering VV-ECMO in adults with ARDS (18,19). It is crucial to determine the acute nature of the pulmonary failure, exclude cardiac and/or other organ failure and verify that the respiratory failure cannot be improved with optimal ventilator management. In the case of VV-ECMO indications, it is imperative to identify any patient characteristics that may prevent VV-ECMO implantation. Thus, before VV-ECMO insertion, a comprehensive echocardiographic examination should be performed, as permitted by the patient’s hemodynamic condition. The use of VV-ECMO support depends on the underlying etiology of respiratory failure. Whereas the incidence of right ventricular (RV) failure has been considerably reduced by the lung protective ventilation strategy in severe ARDS patients, it still remains as high as 25% (20,21). Even if respiratory failure is predominant, the choice of ECMO configuration that would best support the patient is not always simple. VV-ECMO helps resolve hypoxemia and hypercapnia, allowing lower plateau pressures and resulting in reduced pulmonary vascular resistance. This may improve the hemodynamic instability associated with RV failure. Nevertheless, the etiologies of RV failure may not be immediately reversible, and it may be difficult to determine what proportion of the hemodynamic instability can be attributed to the underlying metabolic disturbances. The presence of significant concomitant left ventricular (LV) dysfunction requires the use of VA-ECMO. In this setting, echocardiography plays an essential role in evaluating the degree of residual LV function. From a theoretical standpoint, a thorough examination of the vascular anatomy will assist in determining any potential barriers to cannulation. The femoral and internal jugular veins (IJVs) should be assessed by echography to detect underlying vascular disease, such as deep venous thrombosis, or the presence of a caval filter that may preclude cannula placement. The size of the venous drainage cannula is a determining factor for blood flow in the ECMO circuit; therefore, the insertion in the largest cannula should be attempted. The diameter of the vessels measured by ultrasound may aid in the choice of cannula size (22). From a practical standpoint, this anatomical evaluation is rarely performed in daily clinical practice before VV-ECMO implantation considering the critical state of these patients. The insertion of cannula could be technically challenging in morbidly obese patients. Finally, supplementary information, such as coagulopathy or any contraindications to anticoagulation, should be stated.An Innovative Approach to Treatment
Complex Heart Surgery with International Quality of Care
Minimally Invasive Heart Surgery
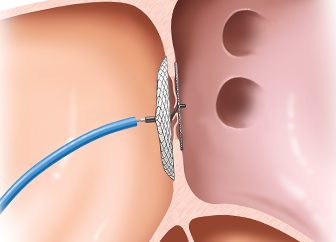
Device Closure of Congenital Defects
Complex Coronary Interventions
Hybrid Procedures with Cardiologists and Surgeons
We Specialise in the Treatment of
- Cardiac Evaluation
- Cardiac Wellness Assessment
- Coronary Interventions
- CABG
- Valve Repair/Replacement Surgery
- Arrhythmia Treatment
- Aortic Aneurysm Surgery
- Device Closure of ASD, VSD, PDA
- Balloon Dilatation of Coarctation
- Valve Stenosis
- RVOT With or Without Stenting
- Congenital Heart Disease Diagnosis
- Resuscitation and Treatment
- Truncus Arteriosus Surgery